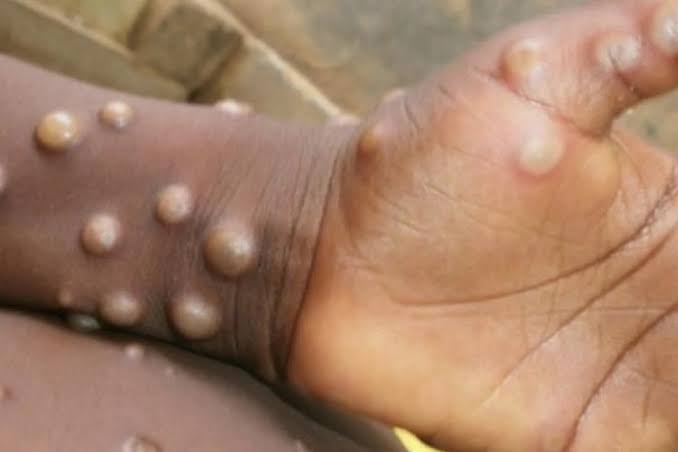
The World Health Organization (WHO) has made a significant breakthrough in the fight against mpox (previously known as monkeypox) by prequalifying the MVA-BN vaccine. This development is expected to increase access to the vaccine, especially in regions hit hardest by the virus, such as parts of Africa.
Prefer to Listen?
The MVA-BN vaccine, produced by Bavarian Nordic, has already been approved in Europe and the U.S. It is primarily intended for adults, with a two-dose schedule that offers up to 82% effectiveness in preventing mpox. However, the WHO has indicated that it can be used off-label for children, pregnant women, and immunocompromised individuals when the benefits outweigh the risks, particularly in outbreak settings.
Dr. Tedros Adhanom Ghebreyesus, WHO Director-General, emphasized that this vaccine approval is a crucial step in addressing the ongoing outbreaks. He urged for rapid scaling up of vaccine procurement and distribution to ensure equitable access in regions facing urgent needs. This call is especially important given that mpox continues to spread, with over 103,000 cases reported globally since the outbreak began in 2022. In 2024 alone, there have been over 25,000 suspected and confirmed cases, mostly concentrated in Africa, where more than 700 deaths have been reported.
The vaccine’s prequalification is expected to expedite the process for countries and international organizations like Gavi and UNICEF to procure and distribute the vaccine more efficiently. This step will also facilitate fast-track regulatory approvals in lower-income countries that rely on WHO’s prequalification for decision-making.
Naijay.com also learned that the WHO recommends administering a single dose of the vaccine in situations where supply is constrained. Even a single dose provides 76% protection, a significant defense for high-risk groups. The MVA-BN vaccine has shown a strong safety profile in both clinical trials and real-world settings, reinforcing its role in the ongoing effort to curb the global spread of mpox.
This prequalification marks a crucial turning point in combating the virus, with hopes that it will significantly reduce transmission rates and save lives in the coming months.